Abstract
Background: There is a lack of data to estimate current life expectancy in adults who have had continual access to best standard of care since birth. Furthermore, the effect of disease-modifying treatments on survival has not been adequately evaluated. Data from the East London Newborn Cohort Study provide information on these outcomes in the setting of the UK National Health Service.
Methods: Inclusion criteria were birth in the London boroughs of Hackney and Tower Hamlets, diagnosis by newborn screening (local programme from 1983 to 2004, and national programme from 2004 onwards), referred and seen at least once in this specialist referral centre. Permission to study all patients in the cohort without individual consent was obtained from the UK National Confidentiality Advisory Group and National Research Ethics Committee. Inclusion was verified by checking against births recorded in national newborn screening dataset. Current status was obtained from hospital records, and for patients no longer attending this service, by contacting their current clinical team, general practitioner and the National Haemoglobinopathy Registry. Survival and factors associated with survival were analysed using Kaplan Meier and Cox Proportional Hazards methods. Subjects were followed from birth to 31st December 2017.
Results: 404 subjects have been enrolled. The median age of surviving patients was 15 (IQ range 9-22) yrs. 202 (50.0%) were female. 177 (43.8%) were being followed in this paediatric service, 98 (24.3%) in this adult service, 40 (9.9%) in other paediatric services, 52 (12.9%) in other adult services; 5 (1.2%) cured with bone marrow transplant, 6 (1.5%) known to be alive but not under active follow-up and 16 (4.0%) lost to follow-up for more than 2 years. Genotypes (%) were HbSS 271 (67.1), HbSC 113 (28.0), HbS beta0 3 (0.7), HbS beta+ 16(4.0), HbSE 1 (0.3). Total years of follow up were 6165.9 (HbSS 4288.8; HbSC 1594.3; other genotypes 282.9). 10 patients (2.5%) have died, as a result of pneumococcal meningitis (2 cases, age 8 and 9 yrs., both HbSS), acute chest syndrome (2 cases, 6 and 19 yrs., both HbSS), acute fat embolism syndrome (1 case, 25 yrs., HbSC), acute R heart failure (1 case, 28 yrs., HbSC), cerebral haemorrhage (1 case, 22 yrs., HbSS), sudden death cause unknown (1 case, 21 yrs., HbSS), recurrent stroke with chronic complications (2 cases, 20 and 25 yrs, both HbSS). In those who died, the median age of death was 22 yrs.
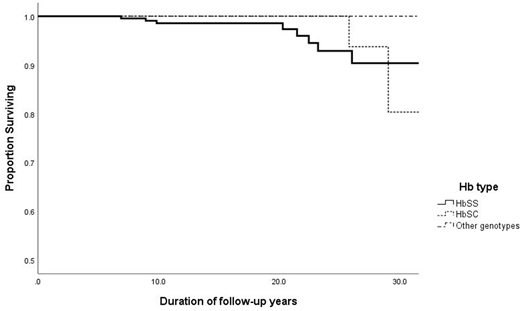
The overall mortality rate for HbSS was 1.9 per 1000 patient yrs, (95% CI 0.9 - 5.0). In follow-up to 20 yrs. of age, and after 20 yrs. of age, it was respectively 1.1 per 1000 patient yrs (95% CI 1-5) and 7.9 (CI 4-25). Overall mortality rate for HbSC was 1.8 per 1000 patient yrs. ( 95% CI 0-5.0). In follow-up to 20 yrs. of age and after 20 yrs. of age it was respectively zero and 11.4 (CI 7-48). The estimated survival (ES) for HbSS at 10 yrs. was 98.6% +/- 0.1 SE and at 25yrs 92.9 % +/- 2.9(SE). For HbSC at age 10 and 25 years ES was 100% (Figure 1).
51 (18.8%) patients with HbSS, and 1 (0.9%) with HbSC have been treated with hydroxyurea (HU) for >6 months for a total of 812.1 patient yrs. on therapy. The median age (IQ range) at start of HU was 16 yrs. (IQ range 11-19). 57 (21.0%) patients with HbSS and 1 (0.9%) with Hb beta+ have been managed with chronic transfusion for >6 months, for a total of 1149.6 patient yrs. on therapy. The median age (IQ range) at start of transfusion was 10 (16-24) yrs. 1 patient (1.9%) died while on HU and 4 (6.9%) while receiving transfusion. Survival modelling, which included treatment as a time dependent covariate, showed that neither genotype, gender, HU or transfusion treatment was associated with better survival.
Conclusions: The study is the first to evaluate survival in a newborn cohort past the age of 20 years. We have confirmed a low mortality rate in childhood, but increased number of deaths in young adults including those with HbSC. This study provides evidence of the benefit of newborn screening and comprehensive care which is accessible at all ages, free of charge. The uptake of HU, particularly at a young age, has been low and a positive effect of HU on survival as described in other studies has not yet been observed in this cohort. Earlier initiation of disease-modifying treatment and longer-term follow-up will be required to provide evidence of treatment effects of HU and other therapies on survival.
Telfer:Bluebird Bio: Honoraria, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Global Blood Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; ApoPharma Inc.: Membership on an entity's Board of Directors or advisory committees; Terumo: Honoraria. Kaya:Novartis: Honoraria; Novartis: Consultancy; AstraZeneca: Consultancy; British Society For Haematology: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal